|
|
Sealing Glass-Ceramics for Planar Solid Oxide Fuel Cells
Literature Review
Alexander Fluegel, 2005, info@glassproperties.com
Abstract: The application of glasses and glass-ceramics for sealing in planar solid oxide fuel cells has been reviewed. The use of element oxides was evaluated systematically. The influences of each component on key sealing properties are discussed.
1. Introduction
Solid Oxide Fuel Cells (SOFC) are ceramic solid-state energy conversion devices that produce electricity by electrochemically combining fuel (e.g. H2, natural gas) and oxidant (e.g. air) gases across an ionic conducting oxide at operating temperatures of ~800°C [1, 2]. The planar SOFC configuration provides a simple manufacturing process and high current densities, but requires hermetic sealing to prevent fuel-oxidant mixing and to electrically-insulate the stack. A suitable sealing material must meet several criteria:
• Chemically stable at ~800°C in oxidizing and reducing wet atmospheres (air, H2)
• Electrically insulating
• Chemically compatible (i.e. must not poison other cell components)
• Sealing at ~900°C, resulting in high bond strength and hermetic seal
• Coefficient of thermal expansion (CTE) ~10-12 ppm/K
• Long-term reliability during high temperature operation, and during thermal cycles to room temperature
The SOFC sealant research can be divided into four successive steps:
1. Examination of the potential components and techniques that may be used for SOFC sealing,
2. Approximation of the composition area,
3. Detailed study of the quantitative influences of each component on the sealing behavior,
4. Chemical and technical process optimization, e.g., chemical surface engineering for controlling crystallization kinetics, long-term stability studies etc.
This literature review is focused on step 1. listed above. Future experimental investigations should be centered on the studies 2. to 4.
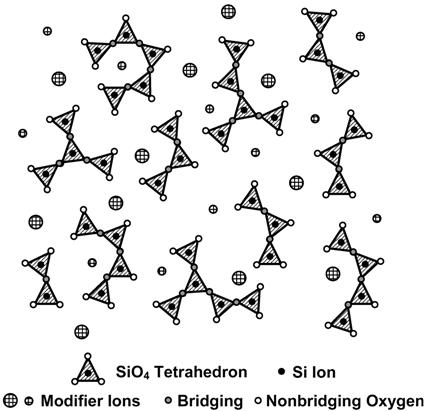
Glass-ceramics, derived from "invert" [3] alkali earth silicate glasses, may be used as sealing materials [4].
The term "invert" glass was introduced by Trapp and Stevels [3], because the traditional network forming oxides SiO2, B2O3, and P2O5 do not form a continuous molecular/ionic network. In fact, the commonly used network modifying oxides are in the majority on the molar basis, i.e., the glasses are inverted structurally compared to soda-lime glasses (see Figure 1).
Alkali glasses containing Li/Na/K are unsuitable for this application, as ion migration in the electric field causes fast seal degradation. It needs to be noted that the diffusion coefficient of the alkali ion Cs+ is reported to be similar to alkali earths ions [5, 6].
In the scientific literature borosilicate glass-ceramics [1, 4, 7, 8, 9, 10, 11, 16, 18], boron-free alkaline earth silicates [12], and phospho-silicate glass-ceramics [13, 14] are suggested for SOFC sealing applications. In this review the emphasis was on borosilicate compositions, because P2O5 increases the crystallization tendency in most silicate glasses more than desired, and because of chemical stability concerns. Furthermore, the phospho-silicates investigated by Larsen et al. [13, 14] exhibit low CTE values of 5-6 ppm/K. Boron-free alkaline earth silicates were not considered because of high viscosities [12, 26].
The short-term goals for the development of a glass-ceramic sealant may be specified as follows: reduced B2O3 concentration for good chemical stability (less than 10 mol%) while maintaining a low sintering and sealing temperature (~900°C), and a high thermal expansion (~12 ppm/K).

Figure 1: Structure of inverted glasses
2. Potential sealing materials and techniques
The requirements allow specific oxide components of a sealing glass-ceramic, which form a systematic pattern in the periodic table of the elements. In Fig. 2 the elements of oxides that may show promising properties in SOFC seals are marked yellow:
Figure 2: Potential oxides for sealing glass-ceramics, derived from [15] (click image to enlarge)
2.1. Glass network former
Besides SiO2 and B2O3, other glass networks former are P2O5, GeO2, TeO2, SeO2, As2O3, Sb2O3, V2O5, etc. Except for SiO2, B2O3, and P2O5, these oxides are reduced by H2 at 800°C [15]. The use of P2O5 may be limited because it increases the crystallization tendency in most silicate glasses. Therefore, for SOFC sealing glass-ceramic application, the network former SiO2 and B2O3 may be preferred.
B2O3 decreases the viscosity and the crystallization tendency of glasses. With increasing B2O3/SiO2 ratio the thermal expansion of sealing glasses increases as shown by Sohn et al. [16]. The disadvantage of high B2O3 concentrations is that it forms volatile compounds with water vapor in the gaseous phase [15, 17] (see Fig. 3) which leads to seal degradation. The properties of B2O3 make it a critical component whose concentration in the seal needs to be evaluated carefully, and if possible the concentration should be decreased.
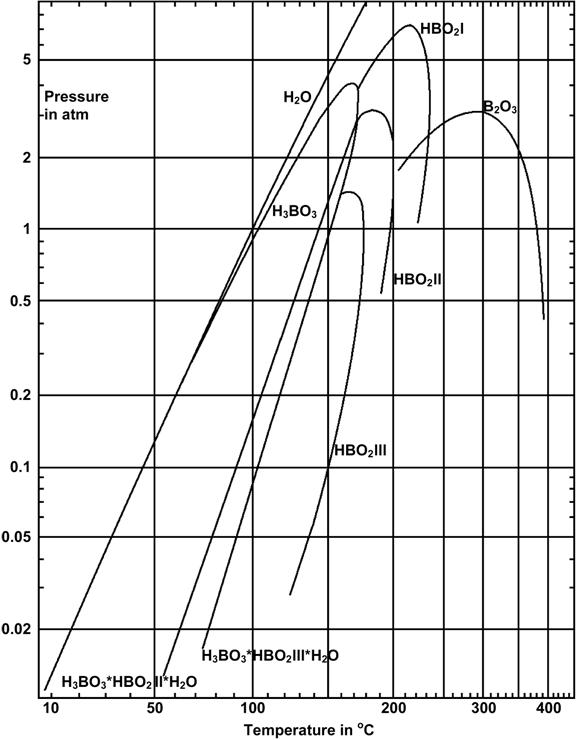
Figure 3: Vapor pressure of saturated solutions in the system B2O3-H2O and decomposition pressure of H3BO3 [15]
2.2. Intermediate glass oxides
Intermediate glass oxides that may be used in sealing glasses are: Al2O3, ZrO2, HfO2, Ta2O5, and ZnO. Substantial amounts of TiO2 may be reduced by H2 at 800°C. Cr2O3 can be oxidized to Cr(VI) and react with BaO to form BaCrO4 [1, 18]. ZnO is reduced by dry H2 at temperatures as low as 300°C, however wet H2 has no influence even at temperatures significantly higher than 300°C [15]. Carbon-containing fuel gases, e.g., methanol or methane, reduce ZnO under wet and dry conditions [15]. ZrO2 and ZnO are known for lowering the thermal expansion of glasses, and ZrO2 increases the viscosity [19, 21]. HfO2 was not considered here because it is very similar to ZrO2 in many of its properties (customarily attributed to the lanthanide contraction which explains the equivalent size of Hf and Zr). Consequently the research on SOFC sealing glass-ceramics was focused on the intermediate oxides Al2O3, Ta2O5, and with reservations ZnO.
2.3. Glass network modifier
For SOFC sealing glass-ceramics the following modifiers may be investigated: alkaline earth oxides, La2O3, Y2O3, rare earth oxides, and Cs2O. Among the alkaline earth oxides, SrO and BaO cause the greatest reduction in viscosity in the glass softening range [19, 20, 21, 32], they increase the CTE [22], and show better glass formation [23] (Figure 5) with lesser phase separation tendency [24] (Figure 4) than do MgO and CaO. Therefore, SrO and BaO should be preferred in sealing glasses over MgO and CaO. The formation of mechanically weak celsian (BaAl2Si2O8, CTE(22-1000°C) = 2.29 ppm/K [25]) has to be avoided.
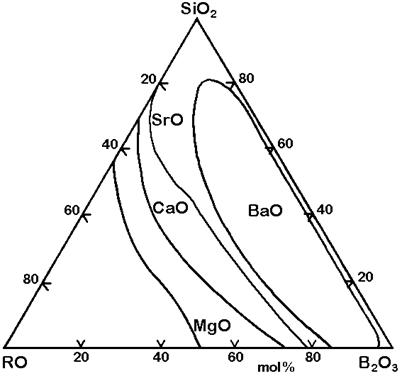
Figure 4: Stable phase separation (immiscibility) in alkali earth borosilicate glasses [24]
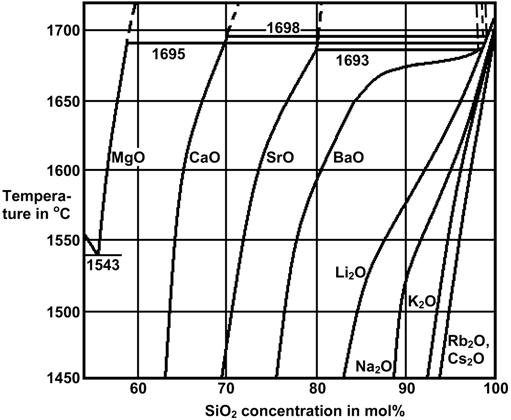
Figure 5: Immiscibility in binary alkali earth silicate glasses [23]
La2O3 is reported to increase the thermal expansion considerably, similar to SrO and BaO [26]. It should be investigated if La2O3 offers advantages over SrO and BaO.
The rare earth oxides and Y2O3 are reported to have similar properties [27]. Glass formation and thermal expansion increase with increasing ionic radius, while viscosity decreases. Therefore, among the rare earth oxides, Nd2O3 and Pr2O3 may be preferred. However the rare earth oxides do not seem to offer advantages compared to SrO and BaO. CeO2 may vary oxidation states in the SOFC environment.
Cs2O is the only alkali oxide that is reported to cause comparable or less ion diffusion than alkali earth oxides [5, 6]. In addition, the Cs+ ion charge is only half of the alkali earth ions, therefore the driving force for Cs+ in the electric field in SOFC is half compared to alkali earth ions. Cs2O is the modifying oxide that leads to the best glass formation among all alkali and alkali earth oxides (e.g. see Figure 5, and Cs2O does not lead to phase separation in ternary alkali-borosilicate glasses as the other alkali oxides do [24]). The disadvantages of Cs2O are chemical stability problems in water, evaporation [15], and its strong basicity. The use of Cs2O in SOFC sealing glass-ceramics may be evaluated compared to B2O3.
2.4. Alternative techniques
An example for SOFC sol-gel derived seals is given by Lewinsohn et al. [28].The disadvantages of the referenced method are that 1) the seal is rigid, i.e., it does not yield to stress at high temperatures, and 2) specific metals with high CTE need to be incorporated for increasing the CTE of the seal. Further materials may be tested.
In addition, seals based on muscovite mica KAl2(Si3Al)O10(OH,F)2 and phlogopite mica KMg3(Si3Al)O10(F,OH)2 were investigated [29, 30, 31]. The K+-ion migration in the electric field was not examined in those materials, which is crucial for their application.
3. Future work
Interesting oxides for SOFC sealing glass-ceramics may include SiO2, B2O3, Al2O3, Ta2O5, BaO, SrO, and CaO as the main components. In future studies about the development of suitable sealing glass-ceramics for solid oxide fuel cells it is recommended to perform studies on the crystallization behavior, since few publications are known in the field. The crystallization behavior significantly controls the kind and the amount of components that may be used in the seal. The investigations about the crystallization behavior may be divided into two parts: 1) Crystallization kinetics during sealing, and 2) Crystallization kinetics and phase composition during long-term application.
Concerning the crystallization behavior during sealing it is crucial to quantify the influences of all components and component-interactions on the crystallization kinetics through scanning electron microscopy (SEM), hot stage microscopy and X-ray diffraction, similar to the work by Lara et al. [12]. In addition, surface-active additives, i.e., sintering aids (e.g. boric acid solutions or sol-gel derived active oxides), can influence the crystallization during sealing significantly, and should therefore be studied. Finally, the phase development during long-term application needs to be analyzed systematically, including the interaction of seal and sealant.
Experimental studies based the this review are published in the article: A. Flügel, M. D. Dolan, A. K. Varshneya, Y. Zheng, N. Coleman, M. Hall, D. Earl, S. T. Misture: "Development of an Improved Devitrifiable Fuel Cell Sealing Glass"; J. Electrochem. Soc., vol. 154, 2007, no. 6, p B601-B608.
4. References
[1] Z. Yang, J. W. Stevenson, K. D. Meinhardt; "Chemical interactions of barium-calcium-aluminosilicate-based sealing glasses with oxidation resistant alloys", Solid State Ionics 160 (2003), p 213– 225
[2] N. Q. Minh: "Ceramic fuel cells"; J. Am. Ceram. Soc., vol. 76 (1993), no. 3, p 563-588
[3] H. J. L. Trapp, J. M. Stevels: "Physical properties of invert glasses"; Glastechn. Ber., vol. 32 K (1959), VI, p 32-52
[4] US patent 6,532,769 (K. D. Meinhardt et al., 2003)
[5] I. A. Ivanov, V. M. Sedov, A. N. Gulin, S. V. Stefanovskii, V. M. Shatkov; Fizika i Khimiya Stekla, vol. 17 (1991), no. 2, p 351
[6] P. J. Melling, C. S. Vempati, A. R. Allnatt, P. W. M. Jacobs: "Tracer diffusion in and electrical conductivity of a natural volcanic glass: rhyolite"; Phys. Chem. Glasses 1981, vol. 22, no. 3, p 49
[7] Sung-Bum Sohn, Se-Young Choi: "Suitable Glass-Ceramic Sealant for Planar Solid-Oxide Fuel Cells"; J. Am. Ceram. Soc., vol. 87 (2004), no. 2, p 254-260
[8] S. Ohara, K. Mukai, T. Fukui, Y. Sakaki, M. Hattori, Y. Esaki: "A new sealant material for solid oxide fuel cells using glass-ceramic"; J. Ceram. Soc. Japan, vol. 109 (2001), no. 3, p 186-190
[9] R. E. Loehman, H. P. Dumm, H. Hofer: "Evaluation of sealing glasses for solid oxide fuel cells"; Ceramic Engineering and Science Proceedings, vol. 23, no. 3, 2002, p 699-710
[10] R. Zheng, S. R. Wang, H. W. Nie, T.-L. Wen: "SiO2-CaO-B2O3-Al2O3 ceramic glaze as sealant for planar ITSOFC"; J. Power Sources, vol. 128 (2004), p 165-172
[11] Z. Yang, G. Xia, K. D. Meinhardt, K. S. Weil, J. W. Stevenson: "Chemical stability of glass seal interfaces in intermediate temperature solid oxide fuel cells"; Journal of Materials Engineering and Performance, vol. 13, no. 3, June 2004, p 327-334
[12] C. Lara, M. J. Pascual, M. O. Prado, A. Durán: "Sintering of glasses in the system RO–Al2O3–BaO–SiO2 (R=Ca, Mg, Zn) studied by hot-stage microscopy"; Solid State Ionics, vol. 170, May 2004, Issues 3-4, p 201-208
http://dx.doi.org/10.1016/j.ssi.2004.03.009 (0.5 MB)
[13] P. H. Larsen, P. F. James: "Chemical stability of MgO/CaO/Cr2O3-Al2O3-B2O3-phosphate glasses in solid oxide fuel cell environment"; J. Mat. Sci., vol. 33 (1998), p 2499-2507
[14] P. H. Larsen, F. W. Poulsen R. W. Berg: "The influence of SiO2 addition to 2MgO - Al2O3 - 3.3P2O5 glass"; Journal of Non-Crystalline Solids 244 (1999) 16-24
[15] Gmelin’s handbook of inorganic chemistry, Verlag Chemie, Weinheim, Germany
[16] Sung-Bum Sohn, Se-Young Choi, Gyeung-Ho Kim, Hue-Sup Song, Goo-Dae Kim: "Stable sealing glass for planar solid oxide fuel cell"; J. Non-Cryst. Solids 297 (2002) 103–112
[17] M. H. V. Fernandes, M. Cable: "Reactive vaporization of sodium tetraborate with water vapour"; Glass Technology, vol. 34, no. 1, February 1993, p 26-32
[18] Z. Yang, K. D. Meinhardt, J. W. Stevenson: "Chemical compatibility of barium-calcium-aluminosilicate based sealing glasses with the ferritic stainless steel interconnect", J. Electrochem. Soc. 150 (8), 2003, A1095-A1101
[19] A. Fluegel, D. A. Earl, A. K. Varshneya, D. Öksoy: "Statistical analysis of viscosity, electrical resistivity, and further glass melt properties", Chapter 9 in: "High temperature glass melt property database for process modeling"; Eds.: Thomas P. Seward III and Terese Vascott; The American Ceramic Society, Westerville, Ohio, 2005, ISBN 1-57498-225-7
[20] G. Tunker, H. Scholze; Glastech. Ber., vol. 55 (1982), no. 4, p 61
[21] A. Fluegel, A. K. Varshneya, D. A. Earl, T. P. Seward, D. Oksoy: "Improved composition-property relations in silicate glasses, part I: Viscosity"; in: "Melt Chemistry, Relaxation, and Solidification Kinetics of Glasses", Ceramic Transactions, vol. 170, Proceedings of the 106th Annual Meeting of The American Ceramic Society, The American Ceramic Society, Westerville, Ohio, 2005
[22]
Y. Hasegawa; Glastech. Ber., 1986,
vol. 59, No. 7, p. 189
Y. Moriguchi, K. Miwa, T. Shibuya; GB Patent No. 1429392 Cl 2 C 03 C 3/08,
3/22, H 01 L 21/316, Abridg. Specif., 1976, No. 4539
[23] Werner Vogel: "Chemistry of Glass"; The American Ceramic Society, 1985
[24] O. V. Mazurin: "Phase separation in glass", North-Holland, 1984
[25] C. H. Drummond III, Ceram. Eng. Pro. 11 (1990), p 1072
[26] G. W. Cleek, C. L. Babcock: "Properties of glasses in some ternary systems containing BaO and SiO2"; National Bureau of Standards Monograph 135, Library of Congress Catalog No. 73-600135, Sept. 1973
[27] J. E. Shelby, J. T. Kohli: "Rare-earth aluminosilicate glasses"; J. Am. Ceram. Soc., vol. 73 (1990), no. 1, p 39-42
J. E. Shelby: "Rare-earths as major components in oxide glasses"; Key Engineering Materials, vol. 94-95 (1994), p 1-42
[28] Lewinsohn, Elangovan; "Development of amorphous, non-oxide seals for solid oxide fuel cells"; Ceramic Engineering and Science Proceedings, v 24, n 3, 2003, p 317-322
[29] Y.-S. Chou, J. W. Stevenson; "Mid-term stability of novel mica-based compressive seals for solid oxide fuel cells", J. Power Sources 115 (2003), p 274–278
[30] Y.-S. Chou, J. W. Stevenson; "Thermal cycling and degradation mechanisms of compressive mica-based seals for solid oxide fuel cells", J. Power Sources 112 (2002), p 376–383
[31] Y.-S. Chou, J. W. Stevenson; "Phlogopite mica-based compressive seals for solid oxide fuel cells: effect of mica thickness", J. Power Sources 124 (2003), p 473–478