|
|
Thermal expansion measurement of glasses
Alexander Fluegel, 2005, info@glassproperties.com
Click here for thermal expansion calculation
Introduction
The thermal expansion is caused by the asymmetry of the amplitude of thermal vibrations in the glass [1]. In turn, the asymmetric vibrations can be related to (a) the chemical bonding and composition, and (b) the temperature and fictive temperature (thermal history). The presence of more loosely bonded chemical units [2], a high temperature [3, 4], and a fast cooling rate [4, 5] increase the thermal expansion. On the other hand, a more coherent network, a low temperature, and annealing lead to low expanding glasses.
Here the following nomenclature will be used:

The CTE is the mean slope of the ![]() L/Lo = f(T) or
L/Lo = f(T) or ![]() V/Vo = f(T) curve within the temperature
interval
V/Vo = f(T) curve within the temperature
interval ![]() T, whereby the linear expansivity
T, whereby the linear expansivity ![]() or the volumetric expansivity
or the volumetric expansivity ![]() is the first derivative of the
is the first derivative of the ![]() L/Lo = f(T) or
L/Lo = f(T) or ![]() V/Vo = f(T) curve over T [6]. The
expansivity is also referred to in the literature as "instantaneous
coefficient of thermal expansion" or "true expansivity" or "true
coefficient of thermal expansion", where the CTE can be called
"mean coefficient of thermal
expansion" [7]. The units of CTE and expansivity are 1 ppm/K = 10 x 10-7
K-1 = 10-4 percent/degree. In general, the expansivity
increases with increasing temperature, which means that the CTE increases as
well with increasing
V/Vo = f(T) curve over T [6]. The
expansivity is also referred to in the literature as "instantaneous
coefficient of thermal expansion" or "true expansivity" or "true
coefficient of thermal expansion", where the CTE can be called
"mean coefficient of thermal
expansion" [7]. The units of CTE and expansivity are 1 ppm/K = 10 x 10-7
K-1 = 10-4 percent/degree. In general, the expansivity
increases with increasing temperature, which means that the CTE increases as
well with increasing ![]() T
and/or if
T
and/or if ![]() T
is reported at higher temperatures [6, 8]. Most glass expansion data in the
literature are stated as the CTEL, for T = 20-300°C or
100-300°C [9]. As estimate it can be assumed that CTEL(20-300°C)
=
T
is reported at higher temperatures [6, 8]. Most glass expansion data in the
literature are stated as the CTEL, for T = 20-300°C or
100-300°C [9]. As estimate it can be assumed that CTEL(20-300°C)
= ![]() (150°C)
and CTEL(100-300°C) =
(150°C)
and CTEL(100-300°C) = ![]() (200°C).
(200°C).
Most investigators publish thermal expansion values in connection with the chemical composition of the investigated glasses and the temperature range of the expansion measurement. Generally, the thermal history is assumed to be insignificant, if annealed samples are used. However, J. P. Joule was the first to report volume changes of glass over forty-five years, later known as the "zero point depression" in precision thermometers [10, 11]. This effect is related to long term arrangements of mobile alkali ions in not sufficiently annealed glasses, and it decreases the thermal expansion. Otto Schott found up to 5% expansion differences between fine annealed and chilled bulk glass [5, 12]. Hence, only well annealed glasses should be used for thermal expansion measurements. Annealing may be performed through heating the specimen about 30°C above the glass transition temperature Tg and subsequnt cooling with a rate of 2 K/min 150°C below Tg [16], followed by further cooling to room temperature in draught-free environment.
In addition it needs to be mentioned that glasses in specific composition ranges tend to phase separation [13] and crystallization [14]. Those effects can result in sudden expansivity changes depending on the thermal history. The findings of Stozharov and Bogatyreva [15] about expansion "jumps" in glasses may be regarded as incorrect for homogeneous and well annealed glasses or categorized as crystallization effect.
Thermal expansion measurement
Thermal expansion measurements on glass are usually performed using push rod dilatometers (single push rod, or differential double push rod) [16, 17]. High precision experiments require the interferometric method [18, 19, 20] which is not discussed here. For push rod dilatometers the sample holder and the push rod can be made of fused silica for measurements up to 700°C, or for higher temperatures polycrystalline alumina may be employed. If fused silica push rods are used one must make sure to clean the surface carefully e.g. with alcohol to avoid devitrivication [16], and direct contact with the hands should be avoided. The expansion calibration must be performed using preferably several standard materials with different expansion in comparison, e.g, the NIST sapphire standard reference material
(SRM) 718, platinum, polycrystalline alumina (Lucalox), fused silica, NIST SRM
731, alumina [21], or similar. The temperature calibration may be done using the ![]() -
-![]() -transition
of quartz at 573°C, pre-calibrated thermocouple calibration
instruments, or ASTM methods [22, 23]. The heating and cooling rates generally used for expansion
measurements are about 2-5 K/min, the sample length 20-100 mm (at least 50000 times the dilatometer resolution), and the
cross-sectional area 10-100 mm2. The push rod load force should not exceed 1 N, acting on the whole cross-section of the sample [16]. A linear alignment between the push rod and sample must be ensured over the whole experiment.
-transition
of quartz at 573°C, pre-calibrated thermocouple calibration
instruments, or ASTM methods [22, 23]. The heating and cooling rates generally used for expansion
measurements are about 2-5 K/min, the sample length 20-100 mm (at least 50000 times the dilatometer resolution), and the
cross-sectional area 10-100 mm2. The push rod load force should not exceed 1 N, acting on the whole cross-section of the sample [16]. A linear alignment between the push rod and sample must be ensured over the whole experiment.
It is not possible to get high quality expansion data during the first run. Even though the first cooling curve shows somewhat better reproducibility than the first heating curve, often the error is still several micrometers in the first run due to irregular settlement of the sample and push rods with temperature change, due to stress relaxation, change of morphology, and since the cooling rate below 300°C depends on the room temperature. Therefore, it is advisable to heat and cool the sample several times below the transition range, without touching the dilatometer or the sample [24]. In most cases, from the second or third cycle onwards, the heating curves above 100°C become reproducible with maximal resolution.
Figure 1 shows an example ![]() L/Lo
expansion chart from an alkaline-free sealing glass. Traditionally, the
dilatometric transition temperature Tg(dil) is determined from the
L/Lo
expansion chart from an alkaline-free sealing glass. Traditionally, the
dilatometric transition temperature Tg(dil) is determined from the ![]() L/Lo
curve as the point of intersection of the tangents below and above the slope
change [24], in Figure 1 it is about 656°C (see Tg measurement). Figure 2 displays the first
deviation of the
L/Lo
curve as the point of intersection of the tangents below and above the slope
change [24], in Figure 1 it is about 656°C (see Tg measurement). Figure 2 displays the first
deviation of the ![]() L/Lo curve from Figure
1, defined as the linear expansivity in Equation (2). The expansivity of most glasses increases with increasing temperature:
L/Lo curve from Figure
1, defined as the linear expansivity in Equation (2). The expansivity of most glasses increases with increasing temperature:
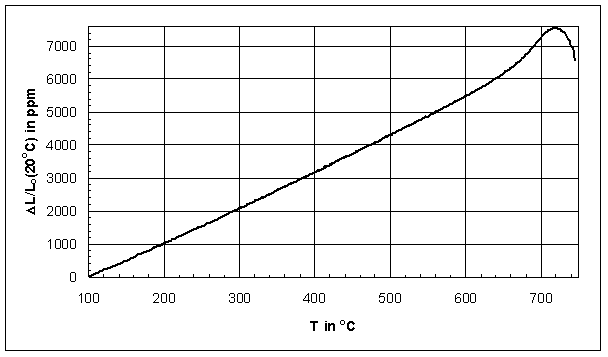
Figure 1: Example ![]() L/Lo curve
L/Lo curve
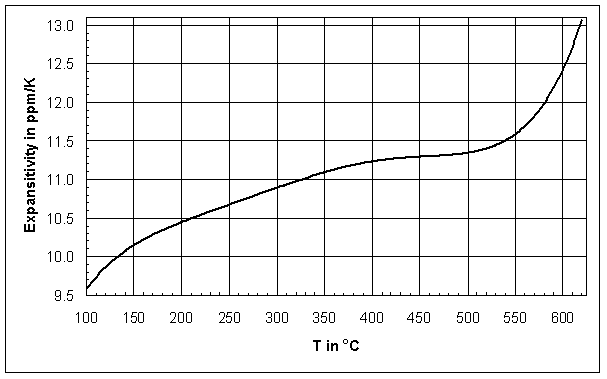
Figure 2: Linear expansivity curve, example from Figure 1
Trough Figure 2 it becomes obvious that the ![]() L/Lo
curve in Figure 1 is not linear at any point, which causes CTE calculations to depend
on the temperature range. In addition, the smooth slope change in Figure 1
makes clear that Tg(dil) determinations by the tangent method do not result in
an exact temperature, but in an approximation of the transition range.
Also it should be noted that the linear expansivity
L/Lo
curve in Figure 1 is not linear at any point, which causes CTE calculations to depend
on the temperature range. In addition, the smooth slope change in Figure 1
makes clear that Tg(dil) determinations by the tangent method do not result in
an exact temperature, but in an approximation of the transition range.
Also it should be noted that the linear expansivity ![]() increases
significantly even 150°C below Tg(dil) of about 656°C,
i.e. CTE data valid up to Tg(dil) might be too high for comparison with other
investigators. Other glasses show a similar expansion behavior, e.g. the
borosilicate glass expansion standard SRM 731 [8].
increases
significantly even 150°C below Tg(dil) of about 656°C,
i.e. CTE data valid up to Tg(dil) might be too high for comparison with other
investigators. Other glasses show a similar expansion behavior, e.g. the
borosilicate glass expansion standard SRM 731 [8].
Possible sources of errors during glass thermal expansion measurements must be minimized:
1. Many expansion measurements are done in the way that the sample is placed into the dilatometer, and the data are recorded right away during heating. This procedure might cause large errors especially at high temperatures, since often the dilatometer pushrods and/or the sample change their positions slightly during the first heating. Some glasses might change their surface layer thickness during the first heating as well. Therefore it is recommended to record the contraction data during controlled cooling, or better to perform several runs until constant readings are obtained.
2.
For most glasses the expansivity
increases sharply within the transition range. However, even 100-150°C
below Tg(dil), ![]() might increase significantly as seen
above. Therefore, if the CTE is reported up to a temperature only slightly
below Tg(dil), the CTE value might be too high for comparison with other
investigators.
might increase significantly as seen
above. Therefore, if the CTE is reported up to a temperature only slightly
below Tg(dil), the CTE value might be too high for comparison with other
investigators.
3. Glass samples must be well annealed, without causing crystallization or phase separation during annealing.
4. The heating and cooling rates generally used for expansion measurements are often 2-5 K/min, the sample length is 20-100 mm, and the cross-sectional area is 10-100 mm2. Significant deviations from those values might result in CTE data that are not comparable.
Volume thermal expansion measurements are performed on glass melts.
References
[1] B. Yates: "Thermal expansion"; Plenum Press, New York - London, 1972, p 33-36
[2] W. L. Konijnendijk, J. M. Stevels: "The linear expansion of borosilicate glasses in relation to their structure"; Verres Réfract., vol. 30, no. 3, 1976, p 371
[3] W. Eitel, M. Pirani, K. Scheel: "Glastechnische Tabellen"; Springer Verlag, Berlin, 1932
[4] G. W. Morey: "The properties of glass"; Reinhold Publishing Corp., New York, 1938, p 264
[5] S. English, W. E. S. Turner: "The heat expansion of soda-lime glasses"; J. Soc. Glass Technol., vol. 3, 1919, p 238
[6] T. A. Hahn: "Thermal expansion of copper from 20 to 800 K - Standard reference material (SRM) 736"; J. Appl. Phys. 41 (1970); p. 5096
[7] ISO 7991: Glass - Determination of coefficient of mean linear thermal expansion, 1987
[8] Standard reference material (SRM) 731, Thermal expansion of borosilicate glass, National Institute of Standards and Technology; Gaithersburg, MD; June 30, 1993
[9] SciGlass 6.5 Database and Information System, 2005
[10] J. P. Joule; Sci. Papers, vol. 1, 1884, p 358
[11] J. T. Krause: "Ultrasonic measurement technique to study thermometric effects in glass"; J. Non-Cryst. Solids, vol. 14,1974, p 32
[12] O. Schott, in: "Vortrag im Verein zur Befoerderung des Gewerbefleisses", 1892, p 161
[13] O. V. Mazurin: "Phase separation in glass"; North-Holland; Amsterdam, New York, 1984
[14] J. H. Simmons, D. R. Uhlmann, G. H. Beall (Eds.): "Nucleation and crystallization in glasses" in "Advances in ceramics"; vol. 4, The American Ceramic Society, Columbus, Ohio, 1982
[15] A. I. Stozharov, V. V. Bogatyreva: "The thermal expansion coefficient and the stability of the refractive index of glasses"; Sov. J. Opt. Technol., vol. 42, no. 10, Oct 1975, p 595
[16] ISO 7991: Glass - Determination of coefficient of mean linear thermal expansion, 1987
[17] ASTM C372-94: Standard Test Method for Linear Thermal Expansion of Porcelain Enamel and Glaze Frits and Fired Ceramic Whiteware Products by the Dilatometer Method, Reapproved 2001
[18] ASTM E289-04: Standard Test Method for Linear Thermal Expansion of Rigid Solids with Interferometry, 2004
[19] ASTM C1300-95: Standard Test Method for Linear Thermal Expansion of Glaze Frits and Ceramic Whiteware Materials by the Interferometric Method, Reapproved 2001
[20] ASTM C539-84: Standard Test Method for Linear Thermal Expansion of Porcelain Enamel and Glaze Frits and Ceramic Whiteware Materials by Interferometric Method, Reapproved 2000
[21] ASTM E2113-04: Standard Test Method for Length Change Calibration of Thermomechanical Analyzers, 2004
[22] ASTM E220-02: Standard Test Method for Calibration of Thermocouples by Comparison Techniques, 2002
[23] ASTM E230-03: Standard Specification and Temperature-Electromotive Force (EMF) Tables for Standardized Thermocouples, 2003
[24] ASTM E1545-00: Standard Test Method for Assignment of the Glass Transition Temperature by Thermomechanical Analysis, 2000
ASTM and ISO standards can be purchased at IHS Global.