|
|
The Mixed-Alkali Effect for the Viscosity of Glasses [1]
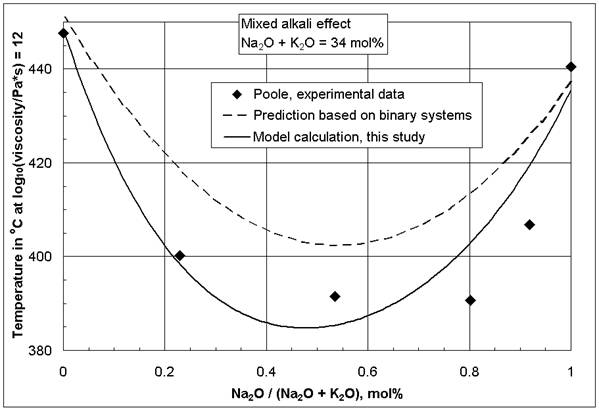
One of the most widely studied component interactions in glasses is the mixed-alkali effect [2-4, 5 (p 132)], especially for properties based on diffusivity such as the electrical conductivity. Controversy exists to the present day about its origin. The mixed-alkali effect also influences the viscosity. Figure 1 illustrates the observation that at constant total alkali concentration ternary mixed-alkali silicate glasses have a lower viscosity than either of the two corresponding binary alkali silicates. This effect can be observed most distinctively at low temperatures, while in the glass melting range its influence is significantly reduced. The mixed-alkali effect is the cause for the stronger influence of K2O compared to Na2O on the viscosity isokom temperatures in the glass softening and annealing range [1]. As the viscosity at low temperatures is significantly reduced, and at the same time at high temperatures not much change occurs, the mixed-alkali effect extends the glass working range. For the viscosity, the mixed-alkali effect also may be observed at low total alkali concentrations, as opposed to the electrical conductivity. For example, Leko [6] found a deep minimum of the viscosity isokom temperatures at log(viscosity, Pa*s) = 10 in ternary mixed-alkali glasses containing 5 mol% Na2O + K2O total, when there was no mixed-alkali effect for the electrical conductivity. In contrast, Nemilov [7] did not observe a mixed-alkali effect for the viscosity in ternary sodium-potassium silicates below 10 mol% total alkali content.

Figure 1: Comparison of viscosity decrease caused by
the mixed-alkali effect in the ternary system SiO2-Na2O-K2O
at log(![]() , Pa*s) = 12.0 after Poole [8] and the models in [1]
, Pa*s) = 12.0 after Poole [8] and the models in [1]
The mixed-alkali effect has a negative (decreasing) effect on viscosity isokom temperatures. The model predictions in [1] describe this behavior well, demonstrated by Figure 1. Therefore, it is the more surprising that all mixed-alkali coefficients Na2O*K2O, Na2O*Li2O, and K2O*Li2O in [1] are not negative, but positive. This can not be explained by linear variable correlation effects [9-11], because the correlation matrix [12] shows that all noteworthy correlations are positive and reduce the values of the mixed-alkali coefficients Na2O*K2O, Na2O*Li2O, and K2O*Li2O.
During the common model fitting technique applied here [9-11] all coefficients are optimized to yield the most simple result with maximum R2 value. An examination of the coefficients in [1] shows that the mixed-alkali effect is not obtained through the coefficients Na2O*K2O, Na2O*Li2O, and K2O*Li2O, but through the coefficients Na2O, (Na2O)2, (Na2O)3, K2O, (K2O)2, (K2O)3, Li2O, (Li2O)2, and (Li2O)3, based largely on experimental data from independent binary alkali silicate systems. The model describes the viscosity behavior in binary alkali silicates and ternary mixed alkali silicates equally well, using mainly the coefficients for binary alkali silicates. Therefore, following the model logic the mixed-alkali effect for the viscosity is not caused by alkali-alkali interactions in the first place but mainly by independent alkali-silica interactions. Alkali-silica interactions cause small additions of alkali oxide to pure silica glass to have a relative stronger influence on the viscosity than large additions. In other words, the mixed-alkali effect is a manifestation of a general non-linear composition-viscosity behavior, well known in binary alkali-silicate glasses.
For additional confirmation of this statement a simple model was developed, based exclusively on all available composition-viscosity data of the binary systems SiO2-Na2O and SiO2-K2O from the SciGlass database [13] listed in [1]. The derived model is given in [1], and the dotted line in Figure 1 shows its application to the mixed-alkali system studied by Poole [8]. It is obvious in Figure 1 that a mixed-alkali effect for the viscosity derives directly from non-linear composition-viscosity behavior of independent binary systems, without alkali-alkali interaction.
Figure 2 shows the non-linear composition-viscosity behavior in binary glasses in one of its extreme cases. The experimental data in Figure 2 were published by Leko et al. [13, 14], and they are well accepted based on comparable values [5 (p 165)] and the structural model by Avramov et al. [15]. The models in [1] can not be applied to the high-silica glasses as in Figure 2; however, in [1] it is shown that also at higher alkali concentrations the composition-viscosity curves in the binary systems SiO2-Na2O and SiO2-K2O are not linear. Additional examples for non-linear composition-property behavior were provided by Mazurin et al. [16] using the large glass property database SciGlass [13].
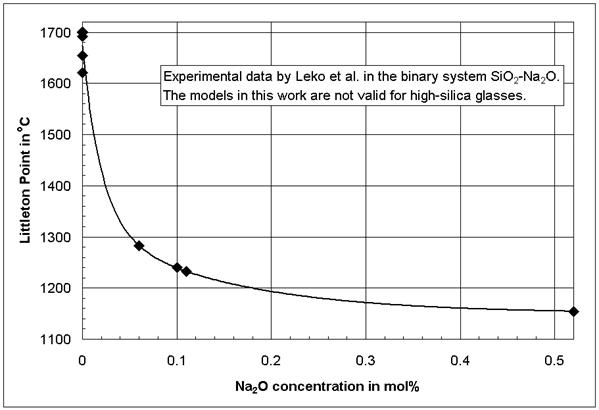
Figure 2: Littleton softening point (isokom
temperature at log(![]() , Pa*s) = 6.6) in the binary glass system SiO2-Na2O
after Leko et al. [14]
, Pa*s) = 6.6) in the binary glass system SiO2-Na2O
after Leko et al. [14]
The positive coefficients for the Na2O*K2O, Na2O*Li2O, and K2O*Li2O could be caused by interactions between two different alkali oxide - silica bonding structures, which have a comparatively weak influence on the viscosity. On the other hand, the mixed-alkali effect for the viscosity is largely based on interactions between alkali oxides and silica, which have a comparatively strong influence on the viscosity, without direct alkali-alkali interference. Alkali-ion interactions in mixed-alkali glasses through ion-pair formation can not be conclusively verified experimentally [17], which lets the modeling efforts of Lyon appear unreliable where a theoretical mixed-alkali compound Na2K2O2 in glass is assumed [18].
According to the global model in [1], the mixed-alkali effect for the viscosity is not a special case that occurs exclusively in mixed-alkali silicate glasses. Many glass components show non-linear composition-viscosity trends. The literature reports mixed alkaline earth [21] (see also Figure 3), mixed-oxide [19, 22], mixed-anion [23], mixed-alkali-water [24], and mixed glass-former [25] effects that even occur in glasses where non-bridging oxygen sites are absent [26]. In addition, mixed mobile ion [27], and similar effects [4, 28] are known for other properties that quite possibly occur also for the viscosity. Naturally, the mixing effects can only be observed as viscosity minima or maxima as long as the influences of the considered components are similar; otherwise, the strong difference between both influences leads to a more or less non-linear composition-viscosity function without extremum if mixing effects are present.
From the MgO*CaO coefficients in [1] it appears that the simultaneous presence of MgO and CaO in a glass decreases viscosity isokoms, especially at low temperatures. It is likely that the negative MgO*CaO coefficients are a manifestation of a mixed-alkaline earth effect that could not be reduced otherwise to MgO and CaO squared and cubic terms [1], based mainly on binary alkaline earth silicates, because those glasses are difficult or impossible to prepare.
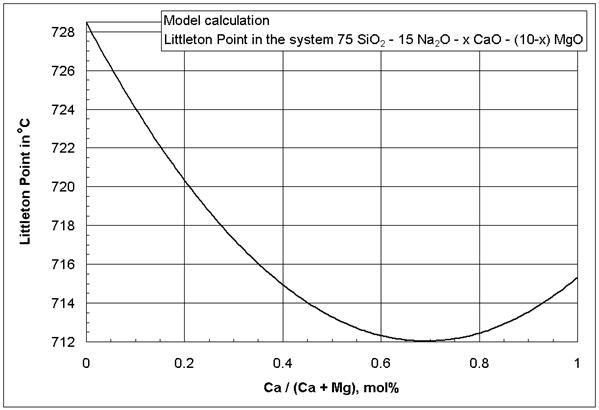
Figure 3: Littleton softening point (isokom
temperature at log(![]() , Pa*s) = 6.6) in the glass system SiO2-Na2O-CaO-MgO,
model calculation. The 95% confidence interval of the model mean in Figure 3 is
2-6oC, depending on the glass composition.
, Pa*s) = 6.6) in the glass system SiO2-Na2O-CaO-MgO,
model calculation. The 95% confidence interval of the model mean in Figure 3 is
2-6oC, depending on the glass composition.
The global models in this study [1] do not explain the cause of the mixed-alkali effect for the viscosity and similar composition-viscosity trends, but they show that the mixed alkali-effect and non-linear composition-viscosity trends in binary glasses mainly have the same origin, which are interactions of individual alkali oxides with glass network formers rather than interactions between alkali oxides. Details of those network former-alkali interactions remain the topic of other research such as by Avramov et al. [15], especially such extreme behavior as seen in Figure 2.
If this hypothesis about the mixed-alkali effect for the viscosity holds true, it should not vanish at low alkali concentrations because at low alkali concentrations the composition-viscosity behavior becomes strongly non-linear as seen in Figure 2. This is in agreement with experimental observations about silicate glasses by Leko [6] who observed the mixed-alkali effect at 5 mol% total alkali content, and in disagreement with observations by Nemilov [7] who did not observe it at 10 mol% total alkali content.
It would be interesting to apply the approach presented here to mixed-alkali effects of other properties, especially the electrical conductivity. For this purpose it needs to be considered that the mixed-alkali effect must have at least two causes with opposite influence. If only one cause existed (glass network weakening) then with decreasing viscosity the conductivity would increase when alkalis are mixed. However, the mixed-alkali effect leads to a decrease of the electrical conductivity (ion mobility reduction) [29]. Glass network weakening by itself leads to an improved ion mobility. This contradiction is recognized but not often discussed or even understood in the literature [2, p 260] because the main focus of mixed-alkali research is centered on the electrical conductivity, i.e., the ion mobility reduction. While concerning the viscosity the mixed-alkali effect appears mainly due to the mixing of two independent binary systems, in the case of the electrical conductivity strong alkali-alkali interactions need to be taken into account. This agrees in principle with Kim et al. [30], who conclude from their experiments that the mixed-alkali effects for the viscosity and electrical conductivity must be considered separately. It is possible that the Na2O*K2O, Na2O*Li2O, and K2O*Li2O interactions in [1] that moderately increase the viscosity, have a strong impeding effect on the alkali ion mobility. If proven as correct, those opposing causes might be the reason why the mixed-alkali effect for the electrical conductivity is not observed at low alkali concentrations; i.e., network weakening and ion mobility reduction cancel each other concerning ion mobility. In this respect it is beneficial to evaluate the experimental findings of Ivanov [31], according to which the conductivity minimum in mixed-alkali germanate glasses not only disappears at low alkali concentrations into a linear function, but beyond this, the curvature slightly reverses from concave to convex. Accordingly, at very low alkali concentrations the mixed-alkali effect for the electrical conductivity could be speculated to occur reversed if the influence of the network weakening on the ion mobility is stronger than the ion mobility reduction caused by alkali-alkali interaction.
REFERENCES
[1] A. Fluegel: "Glass Viscosity Calculation Based on a Global Statistical Modeling Approach"; (PDF, 3 MB), Glass Technol.: Europ. J. Glass Sci. Technol. A, vol. 48, 2007, no. 1, p 13-30.
[2] J. O. Isard: "The mixed alkali effect in glass"; J. Non-Cryst. Solids (1969), vol. 1, no. 3, p 235-261.
http://dx.doi.org/10.1016/0022-3093(69)90003-9
[3] D. E. Day: "Mixed alkali glasses - their properties and uses"; J. Non-Cryst. Solids (1976), vol. 21, no. 3, p 343-372.
http://dx.doi.org/10.1016/0022-3093(76)90026-0
[4] W. C. LaCourse, A. N. Cormack: "Structural influences on the mixed alkali effect in glasses"; Transactions of the American Crystallographic Association (1991), vol. 27, p 211-224 (Proceedings of the Symposium on The Structural Chemistry of Silicates, Jul 22-24 1991, Toledo, OH, USA).
[5] H. Scholze: "Glass - Nature, Structure and Properties"; Springer-Verlag (1991), ISBN 0-387-97396-6.
[6] V. K. Leko; Inorgan. Mater. (1967), vol. 3, p 1645.
[7] S. U. Nemilov; Zh. Prikl. Kim. (Leningrad) (1969), vol. 42, p 55.
[8] J. P. Poole, M. Gensamer: "Systematic study of effect of oxide constituents on viscosity of silicate glasses at annealing temperatures"; J. Am. Ceram. Soc. (1949), vol. 32, no. 7, p 220-229.
J. P. Poole: "Low-temperature viscosity of alkali silicate glasses"; J. Am. Ceram. Soc. (1949), vol. 32, no. 7, p 230-233.
[9] A. Fluegel, D. A. Earl, A. K. Varshneya, D. Öksoy: "Statistical analysis of viscosity, electrical resistivity, and further glass melt properties", Chapter 9 in: "High temperature glass melt property database for process modeling"; Eds.: T. P. Seward III and T. Vascott; The American Ceramic Society, Westerville, Ohio (2005), ISBN: 1-57498-225-7.
http://www.ceramics.org/glassmelt/
http://www.osti.gov/bridge/purl.cover.jsp?purl=/809193-hMVo0M/native/
[10] A. Fluegel, D. A. Earl, A. K. Varshneya, T. P. Seward: "Density and thermal expansion calculation of silicate glass melts"; Proceedings CD of the 79. Glastechnische Tagung, Wuerzburg, Germany, May 23-25, 2005.
http://glassproperties.com/density/
[11] A. Fluegel: "Application of Statistical Property Analysis for Targeted Glass Research and Process Modeling - Tutorial"; submitted for publication
http://glassproperties.com/principle/
[12] Viscosity calculation program, http://glassproperties.com/viscosity
[13] SciGlass 6.5 Database and Information System (2005).
[14] V. K. Leko, N. K. Gusakova, E. V. Meshcheryakova, T. I. Prokhorova: "The effect of impurity alkali oxides, hydroxyl groups, Al2O3, and Ga2O3 on the viscosity of vitreous silica"; Glass Phys. Chem. (1977), vol. 3, no. 3, p 204-219.
V. K. Leko, E. V. Meshcheryakova, N. K. Gusakova, R. B. Lebedeva; Opt. Mekh. Prom. (1974), no. 12, p 42.
[15] I. Avramov, C. Rüssel, R. Keding: "Effect of chemical composition on viscosity of oxide glasses"; J. Non-Cryst. Solids (2003), vol. 324, p 29-35.
[16] O.V. Mazurin, Yu. Gankin: "About testing the reliability of glass property data in binary systems"; J. Non-Cryst. Solids (2004), vol. 342, p 166-169.
http://www.sciglass.info/Publications/MazurinGankin1.pdf
http://dx.doi.org/10.1016/j.jnoncrysol.2004.06.012
O.V. Mazurin, Yu. Gankin: "Determination of the most reliable glass property values by the SciGlass information system"; Proceedings CD of the XX International Congress on Glass, Kyoto, Japan, Sept. 27 - Oct. 1, 2004.
http://www.sciglass.info/Publications/MazurinGankin2.pdf
O. V. Mazurin: "Glass properties: compilation, evaluation, and prediction"; J. Non-Cryst. Solids (2005), vol. 351, no. 12-13, p 1103-1112.
http://www.sciglass.info/Publications/Mazurin2.pdf
http://dx.doi.org/10.1016/j.jnoncrysol.2005.01.024
[17] C. D. Hanson, T. Egami: "Distribution of Cs+ ions in single and mixed alkali silicate glasses from energy dispersive X-ray diffraction"; J. Non-Cryst. Solids, 1986, vol. 87, no. 1-2, p 171-184.
http://dx.doi.org/10.1016/S0022-3093(86)80077-1
[18] K. C. Lyon: "Prediction of the Viscosities of Soda-Lime Silica Glasses"; J. Res. Nat. Bur. Standards A, Physics and Chemistry (1974), vol. 78A, no. 4, p 497-504.
[19] G. Berg, A. Ludwig: "Mixed oxide effect in an ion-exchanged glass"; J. of Non-Cryst. Solids (1994), vol. 170, no. 1, p 109-111.
http://dx.doi.org/10.1016/0022-3093(94)90111-2
[20] G. Tomandl, H. A. Schaeffer: "The mixed-alkali effect - a permanent challenge"; J. Non-Cryst. Solids, 1985, vol. 73, p 179-196.
http://dx.doi.org/10.1016/0022-3093(85)90345-X
G. Tomandl, H. A. Schaeffer in: "Non-Crystalline Solids", ed. by G. H. Frischat; Trans Tech Publications, Bay Village, Ohio, 1977, p 480.
[21] Ki-Dong Kim, Jong-Hee Hwang: "Influence of BaO/(SrO+BaO) on some thermal properties of R2O-RO-SiO2 glasses for plasma display panel substrate"; Glass Sci. Technol, Glastechnische Berichte (1999), vol. 72, no. 12, p 393-397.
[22] R. Waesche, R. Brueckner: "Tg, UV-transparency and mixed oxide effect of ultrapure ternary alkaline earth-metaphosphate glasses"; J. Non-Cryst. Solids (1989), vol. 107, no. 2-3, p 309-315.
http://dx.doi.org/10.1016/0022-3093(89)90477-8
[23] J. E. Shelby, R. L. Ortolano: "Properties and structure of NaF-Na2O-B2O3 glasses"; Phys. Chem. Glasses (1990), vol. 31, no. 1, p 25-29.
[24] J. E. Shelby, G. L. McVay: "Influence of water on the viscosity and thermal expansion of sodium trisilicate glasses"; J. Non-Cryst. Solids (1976), vol. 20, no. 3, p 439-449.
http://dx.doi.org/10.1016/0022-3093(76)90124-1
[25] P. S. S. Prasad, A. N. D. Rani, S. Radhakrishna: "Mixed glass former effect in AgI-Ag2O-V2O5-P2O5 quaternary amorphous solid electrolytes"; Materials Chemistry and Physics (1990), vol. 25, no. 5, p 487-499.
[26] J. C. Lapp, J. E. Shelby: "The mixed alkali effect in sodium and potassium galliosilicate glasses I. Glass transformation temperatures"; J. Non-Cryst. Solids (1986), vol. 84, no. 1-3, p 463-467.
http://dx.doi.org/10.1016/0022-3093(86)90810-0
J. C. Lapp, J. E. Shelby: "The mixed alkali effect in sodium and potassium galliosilicate glasses II. DC electrical conductivity"; J. Non-Cryst. Solids (1986), vol. 86, no. 3, p 350-360.
http://dx.doi.org/10.1016/0022-3093(86)90023-2
[27] S. Ghosh, A. Ghosh: "Ion dynamics and mixed mobile ion effect in fluoride glasses"; J. of Appl. Phys. (2005), vol. 97, no. 12, p 123525.
http://dx.doi.org/10.1063/1.1939084
[28] H. C. Brook, A. V. Chadwick, J. F. W. Mosslemans, G. N. Greaves: "EXAFS study of a 'mixed-alkali' type effect in sodium-calcium borate glass"; Radiation Effects and Defects in Solids (1999), vol. 150, no. 1-4 pt. 2, p 795/403 - 799/407.
[29] J. Swenson, S. Adams: "Mixed alkali effect in glasses"; Phys. Rev. Lett. (2003), vol. 90(15), p 155507.
[30] Ki-Dong Kim, Seung-Heun Lee: "Viscosity behavior and mixed alkali effect of alkali aluminosilicate glass melts"; Nippon Seramikkusu Kyokai Gakujutsu Ronbunshi / Journal of the Ceramic Society of Japan (1997), vol. 105, no. 1226, p 827-832.
[31] A. O. Ivanov; Soviet Phys.-Solid State (1964); vol. 5, p 1933.
Data also reported by Isard [2].